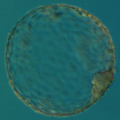
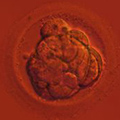
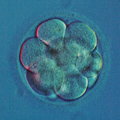
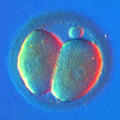
Aneuploidy Testing
Why do genetically normal parents select PGD?
Genetically normal parents produce genetically abnormal embryos in great numbers, and the evidence for this is disturbingly abundant:
- Thousands of babies with Down Syndrome are born in the US every year. About the same number of babies with lesser-known chromosomal disorders (Kleinfelter's, Turner's, Patau's, Edward's) are also born to genetically normal parents.
- At least 15-20% of all human fetuses end up as detectable spontaneous abortions. About half of these embryos abort because they are chromosomally abnormal. A chance in the 7-10% range for miscarriage due to genetic abnormalities is a serious risk, but this number still does not quite reveal the urgency for PGD.
- Failed pregnancies. This happens to every couple trying to conceive. According to some estimates, over 75% of all human conceptions abort before the first missed menstrual cycle. Thanks to PGD we know why this happens. By being able to test human embryos at the very first stages of development PGD reveals the magnitude of the pool of genetic abnormalities generated by humankind. On average, more than half of the embryos of an IVF patient in her mid- to late-thirties are genetically abnormal, or aneuploid. Nature, it seems, found an extremely wasteful, but probably the easiest, way of dealing with genetic abnormalities. Athough generated in large numbers, most genetically abnormal conceptions perish prior to or shortly after implantation.
During an IVF cycle, when so much hope, efforts, and financial investments go into a single attempt, selection should be at the level of individual embryos, not failed pregnancies! By identifying aneuploid embryos, PGD insures that only those embryos which have a chance to establish a normal pregnancy are transferred.
How is it possible that genetically abnormal embryos are generated by genetically normal parents?
Mitotic errors (mistakes in chromosome segregation during routine cell division) occur constantly in our bodies, but the resulting abnormal cells are quickly recognized and eliminated by the immune system. During the first five days of development, the human embryo cleaves and transforms from a single-cell zygote into a blastocyst with hundreds of cells. At these stages, however, the embryo does not have an immune system yet, and genetically abnormal cells are free to compete with normal cells for the privilege of building a baby. A single mitotic error during early cleavage may result in an embryo with a large proportion of genetically abnormal cells.
Surprisingly, the majority of chromosomal abnormalities are generated prior to fertilization, even before an embryo has a chance to establish itself as a new genetic entity. These are the errors of oocyte maturation, which can be addressed by PGD.
How do these abnormal oocytes originate? Why do older women have so few pregnancies and so many abnormal babies?
In the 19th century it was noticed that babies with mongolism (presently recognized as Down's Syndrome, or trisomy for chromosome 21) are mostly last-born children in the family. In 1930, the maternal age effect was shown to be the critical factor: women over 35 years of age gave birth to over half of all babies with Down's Syndrome, although that group accounted for only 15% of all births. The odds of giving birth to a baby with Down's Syndrome increases exponentially with the woman's age. For a 25-30 year old woman the chance is 1 in 1000, but for a 45-year-old woman it skyrockets to 1 in 32.
As it usually happens in biology, the reality behind this phenomenon is rather complicated. Human oocytes (eggs) are well protected from the insecurities of the outside world deep inside the mother's body. Oocytes are first surrounded by the eggshell (called the zona pellucida) then by layers of cells whose only function is oocyte nurturing. In its turn, the major function of the ovaries is oocyte storage, growth and maturation. There are good reasons for all these safety measures. Oocytes are perhaps the most important cells in the female body, and they have to be kept safe and viable for a very long time. Like nerve cells, oocytes do not regenerate, and their population is never replenished. The last new oocyte is formed in the ovary at the 7th month of prenatal development, before a baby girl is even born. Then, for decades, oocytes are stored under the most optimal conditions, but they cannot be fully protected from the passage of time. A 40-year old oocyte, although well protected from the outside world, has been affected by a lifetime of body fevers, distresses, drugs, etc.
Mechanisms underlying genetic maturation of a human oocyte, or meiosis, are very complex but the result of this process is the reduction by half the number of chromosomes the oocyte initially had in its "immature" state. Out of 46 human chromosomes each woman has 22 pairs of so-called autosomes and two sex determination chromosomes: X+X. Men also have 44 autosomes, one X chromosome and one tiny Y chromosome. Autosomes do not have any interesting names, like X or Y, and are known by their number. Thus, each human cell has two chromosomes No.1, two chromosomes No.2, ..., two chromosomes No.22, two chromosomes X (girls) or one X plus one Y (boys).
During meiosis, the oocyte discards its "second set" of 23 chromosomes and after that it is ready to be fertilized by a sperm, which will introduce its own, paternal set of 23 chromosomes. The resulting embryo will have a restored set of 46 chromosomes, 23 maternal and 23 paternal.
Any error made during oocyte maturation will be lethal for the resulting embryo. Let us take chromosome 21 as an example. If, by mistake, the oocyte gets rid of not just one, but of both of its chromosomes 21, the resulting embryo will end up with only one chromosome 21, introduced by a spermatozoon. This condition is called monosomy 21. Monosomy of any other autosome is generally lethal in humans, but a few babies with monosomy 21 have survived beyond birth, with severe abnormalities. No monosomies or nullisomies (embryos with both chromosomes missing) of any other chromosome have ever been found in newborns or in the aborted material. However, these abnormalities do exist in preimplantation embryos, but such embryos perish at the earliest stages of embryo development. These abnormalities "just" add to the statistics of failed pregnancies and failed IVF cycles.
If, on the other hand, an oocyte makes the opposite mistake and fails to extrude one chromosome 21 (while extruding the second set of every other chromosome), the resulting embryo will end up with one extra chromosome 21 - this condition is called trisomy 21. Most of these conceptions (about 75%) will be spontaneously aborted, but those surviving to birth will be the babies with Down's Syndrome, the most common genetic defect in newborns. Other autosomal anomalies with any significant frequency among newborns are trisomies 13 (Patau's Syndrome) and 18 (Edward's Syndrome). Trisomy of any other autosome is lethal at the early stages of prenatal embryo development.
Meiotic error may also cause an abnormal number of sex chromosomes. Per each 100,000 recognized human pregnancies, around 1,500 will be aborted due to an abnormal number of sex chromosomes. Out of the same 100,000 human pregnancies, about 100 boys will be born with Kleinfelter's Syndrome (instead of XY will have XXY set of sex chromosomes) and around 50 girls will be born with Turner's Syndrome (instead of XX will have X_, or XXX).
Apart from chromosomes 13, 18, 21, X, and Y, the only other chromosomes noticeably affecting the outcome of established human pregnancies are chromosomes 16 and 22. Trisomy 16 is found in one in every 11 spontaneous abortions and trisomy 22 is found in one out of every 35 spontaneous abortions. Embryos with trisomy 16 or 22 never reach term, but they affect the outcome of established pregnancies and add to the list of failed IVF cycles.